Daré Bioscience to acquire Microchips Biotech and its drug delivery technology
Daré Bioscience, a clinical-stage bio-pharmaceutical company, today announced its acquiring Microchips Biotech, another biotech company that developed proprietary microchip-based implant can store and release precise doses of drugs over months and years. The total amount of the deal was not disclosed. The deal is subject to regul
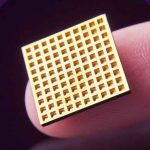
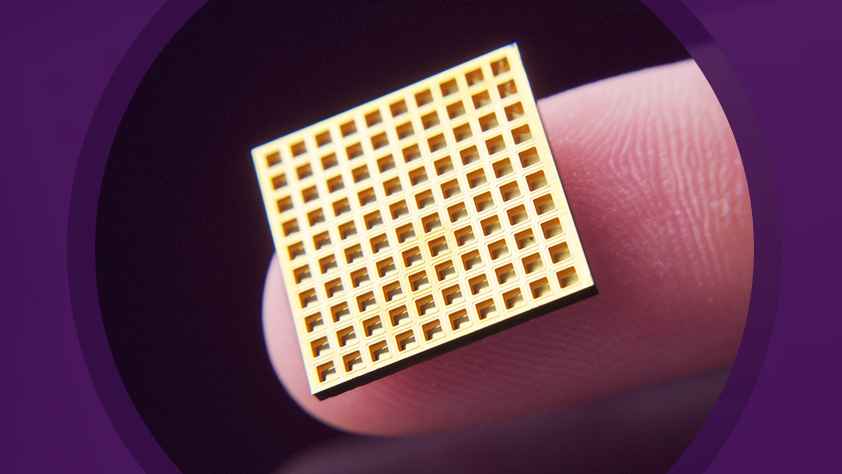
As part of the deal, Daré will acquire Microchips’ innovative, drug delivery technology. The technology, which has been validated in a first-in-human clinical study in osteoporosis patients, is designed to store and precisely deliver hundreds of therapeutic doses over months or years in a single implant. The implant is intended to be operated by the patient to deliver medication on demand or on a pre-determined schedule that can be activated or deactivated wirelessly, as required.
The Lexington, MA-based Microchips Biotech was founded in 1999 by John Santini, Michael Cima, and Terrance McGuire. Its proprietary microchip-based implant can store and release precise doses of drugs over months and years. The implant, which is placed under the skin by a trained physician during a simple office procedure using local anesthesia, can be wirelessly activated or deactivated by a physician or patient, without requiring removal. In addition, physicians can wirelessly modify the frequency or dose of the drug to meet the individual needs of each patient.
The microchip-based implant, originally developed at the Massachusetts Institute of Technology by renowned researchers Robert Langer, Ph.D. and Michael J. Cima, Ph.D., is protected by 98 patents granted and 19 patent applications pending. Microchips, with the support of the Gates Foundation in the form of approximately $17.9 million in grant funding to date, has been developing an implantable long-acting, reversible contraceptive application of the technology, which, if successful, will provide women with unparalleled control over the management of their fertility, which can be individually timed to meet her family planning goals and objectives.
“Integrating the two companies creates a unique opportunity to continue the product and clinical development of this important new drug delivery technology platform with the support of the Bill & Melinda Gates Foundation,” said Sabrina Martucci Johnson, President and CEO of Daré Bioscience. “The addition of this user-controlled, long-acting reversible contraceptive opportunity to Daré’s pipeline, which already includes the novel monthly, hormone-free contraceptive Ovaprene, will uniquely position Daré with two of the potentially most disruptive innovations in the contraceptive category. We look forward to announcing topline data from our Ovaprene postcoital test clinical trial, a pre-pivotal study to support product registration.”
“We are tremendously excited to bring this new, potentially game-changing platform to Daré’s compelling pipeline of women’s health innovations,” said Cheryl R. Blanchard, Ph.D, President and CEO of Microchips Biotech. Dr. Blanchard will be joining Daré’s board of directors post-closing.
“I am excited to contribute to Daré’s mission to advance innovative products for women’s health,” continued Dr. Blanchard. “New and innovative contraceptive options that better meet the needs of women around the world are crucial. The Microchips technology holds immense promise to provide women with a sophisticated and flexible contraceptive product that adapts to their individual family planning needs. It also has the potential to provide critical drug delivery solutions that address significant unmet needs in treating a number of chronic diseases.”