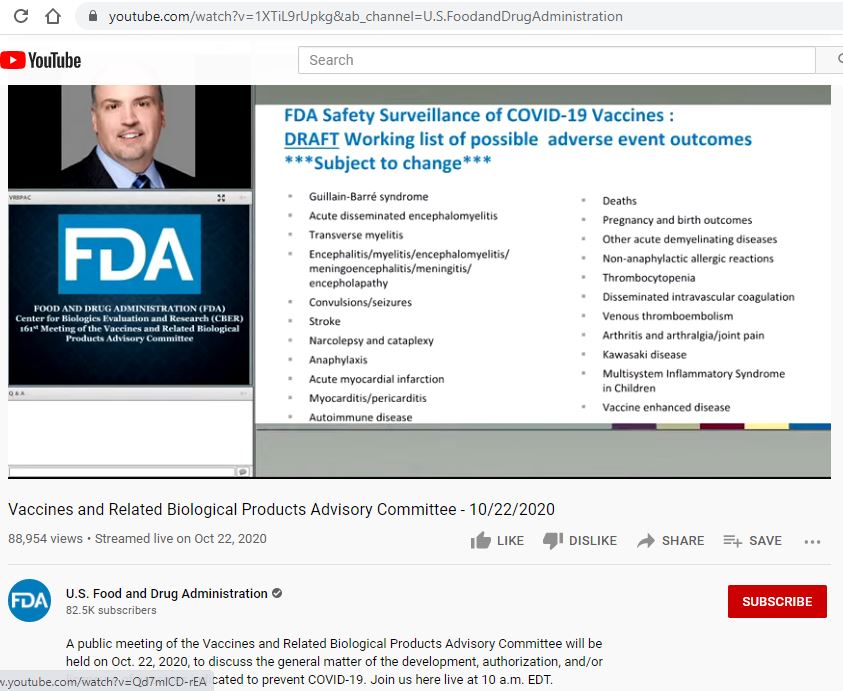
FDA knew about the rare Guillain–Barré syndrome as early as October 2020. A slide from “Draft working list of possible adverse event outcomes” presentation shows

On Monday, the U.S. Food and Drug Administration (FDA) issued warnings about the rare nerve syndrome to the Johnson & Johnson’s coronavirus vaccine can lead to an increased risk of a rare neurological condition known as Guillain–Barré syndrome.
The Guillain–Barré syndrome is a rare autoimmune disease disorder in which the immune system attacks the peripheral nervous system, paralyzing parts of the body. FDA to add a new warning that Johnson & Johnson’s vaccine can lead to an increased risk of a rare neurological condition known as Guillain–Barré syndrome. The Centers for Disease Control and Prevention (CDC) is said to have received about 100 preliminary reports of Guillain-Barré following the one dose of the J&J vaccine.
In a letter addressed to Janssen Biotech, which was also posted on the FDA website, the agency said:
“This letter is to notify you that we have reviewed your requested changes and data to support revisions to your Authorized EUA Fact Sheets, as well as FDA-required changes to include new information about Guillain-Barré syndrome and that your request is granted.”
In the letter, the FDA also added new warnings about Thrombosis with Thrombocytopenia and Guillain-Barré Syndrome.’ In the summary section, the FDA said: “The following was added to this section: “Severe allergic reactions (including anaphylaxis), thrombosis with thrombocytopenia, Guillain-Barré syndrome, and capillary leak syndrome have been reported following administration of the Janssen COVID-19 Vaccine during mass vaccination outside of clinical trials.”
Immediately after the announcement came out, many were wondering why the FDA is just reporting these side effects considering that millions of Americans have already been vaccinated with the J&J vaccines.
As it turned out, the FDA knew about the rare Guillain–Barré syndrome and other potential side effects as early as late last year. On October 22, 2020, a public meeting of the Vaccines and Related Biological Products Advisory Committee was to discuss the general matter of the development, authorization, and/or licensure of vaccines indicated to prevent COVID-19.
One of the presentations discussed during the meeting was a briefing titled: “FDA Safety Surveillance of COVID-19 Vaccines: a DRAFT working list of possible adverse event outcomes. ***Subject to change***.”
During the briefing, the presenter was not able to access his other computer and a third part was advancing the slides for his presentation. At exactly the 2:33:40 mark, the presenter accidentally lets the slide flash on the screen.
As you can see from the “DRAFT working list of possible adverse event outcomes,” the vaccine has the potential to cause any of the following adverse event outcomes including Guillain-Barré Syndrome, transverse myelitis, autoimmune disease, stroke, thrombocytopenia, and even deaths.
The screenshot below is from a “Slide’ presented during the FDA advisory meeting for the Vaccine posted on YouTube. As can be seen from the presentation, the presenter did not intend for this slide to be made public as it was only visible for less than a second.
The slide is exactly at the 2:33:40 mark. To view the video, click on the sprocket wheel next to the “CC” on the right bottom of the video and change the video playback speed to .25. Then the slide should be visible as it flashes for less than one second.
Below is the link to the video