CDC latest vaccination data shows that 3,150 people had life-threatening reactions after taking Pfizer vaccine

It’s been a while since we looked at the Centers for Disease Control and Prevention (CDC) website for the latest updates on the ongoing vaccination effort. According to the CDC, about 10.3 million people have received at least one dose of a COVID-19 vaccine. Of that number, at least 541,000 Americans have been fully vaccinated as of Jan. 12, according to a New York Times survey of all 50 states.
As noted last week, the United States seems to be having great success with the rollout of vaccines that began in December of 2020. There have been zero deaths directly associated with people who took the vaccine. However, there is now a new report of Anaphylaxis (a serious, life-threatening allergic reaction) from the CDC based on vaccination data collected from both the U.K. and the U.S.
Are We Ready For Life-Threatening Reactions (Anaphylaxis) Resulted from Taking COVID-19 Vaccination?
According to the CDC’s latest vaccination data posted on an 8-page report available on the agency’s website, CDC report shows that 3,150 people vaccinated in one day are “unable to perform normal daily activities, unable to work” after taking the Pfizer vaccine. The CDC said that these people required care from doctors or health care professionals to treat severe allergic reactions.
The revelation, which was buried on page 6 of the 8-page report, was disclosed as part of the data in the “ACIP COVID-19 Vaccines Work Group.” The implications of this finding could be disastrous if this data is scaled up on a global level.
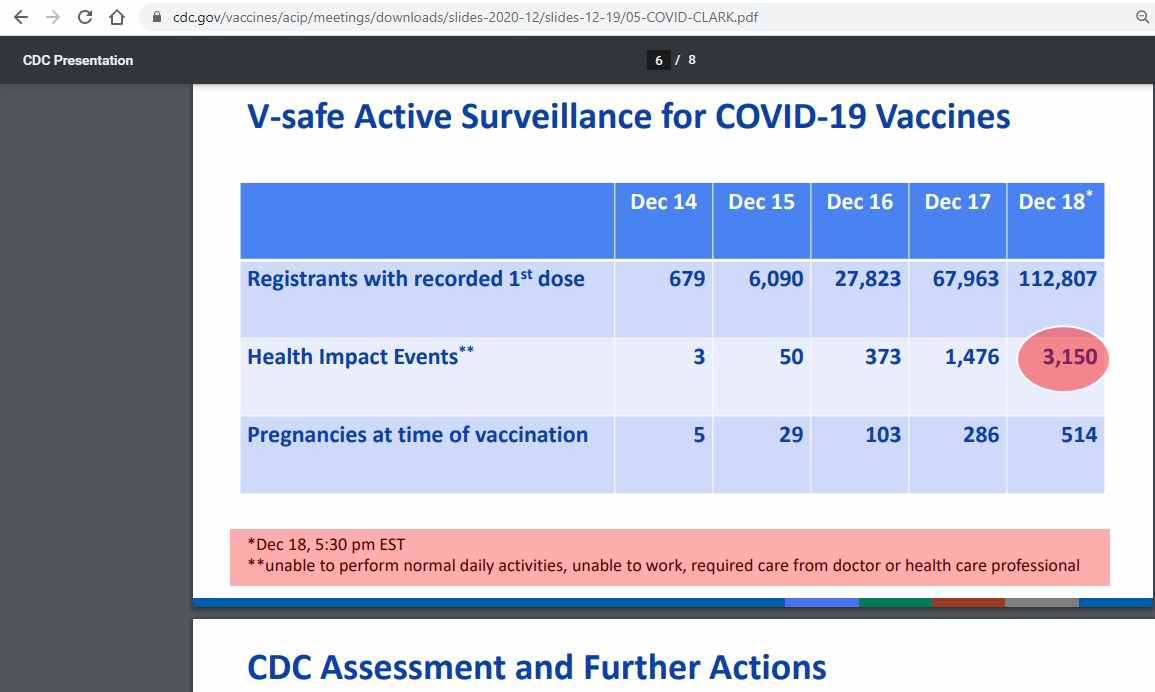
Source: CDC website
On December 8, 2020, the UK initiated vaccination with the Pfizer-BioNTech COVID-19 vaccine. The following day, UK authorities confirmed 2 cases of a severe allergic reaction (i.e. anaphylaxis) after vaccination.
As part of the December 19, 2020 report titled, “Anaphylaxis Following m-RNA COVID-19 Vaccine Receipt,” the CDC and health authorities in the UK looked at anaphylaxis in the UK following COVID-19 vaccination.
Health authorities in the UK found that:
“prescribing information for both Pfizer-BioNTech and Moderna COVID-19 vaccines contains information on anaphylaxis including severe allergic reaction (anaphylaxis) to any component of the vaccine is a contraindication to vaccination and an appropriate medical treatment used to manage immediate allergic reactions must be immediately available in the event an acute anaphylactic reaction occurs following administration of the vaccine.”
The severe allergic reaction (anaphylaxis) is not limited to the United Kingdom alone. On December 18, 2020, the CDC also identified 6 case reports of anaphylaxis following the Pfizer-BioNTech vaccine meeting Brighton Collaboration criteria for anaphylaxis.

Source: CDC website
The CDC concluded the report with the following:
“Persons with anaphylaxis following COVID-19 vaccination should not receive additional doses of COVID-19 vaccine“

Source: CDC website




