FDA adds heart inflammation warnings to Moderna and Pfizer COVID-19 mRNA vaccines
And so it begins. After reports of hundreds who suffered rare heart inflammation following Pfizer and Moderna COVID-19 mRNA vaccines early this month, The U.S. Food and Drug Administration (FDA) added a warning about the risk of developing heart inflammation to information about the Moderna and Pfizer COVID-19 vaccines.
On Friday, the FDA announced “revisions to the patient and provider fact sheets for the Moderna and Pfizer-BioNTech COVID-19 vaccines regarding the suggested increased risks of myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the tissue surrounding the heart) following vaccination.”
The sudden announcement from the FDA came after the Centers for Disease Control and Prevention (CDC) said that “evidence grows stronger that Covid vaccine is linked to a heart issue called myocarditis.” In another report from NBC News, citing the CDC, the media outlet wrote:
“A higher-than-usual number of cases of a type of heart inflammation has been reported following Covid-19 vaccination, especially among young men following their second dose of an mRNA vaccine, the Centers for Disease Control and Prevention said Thursday.”
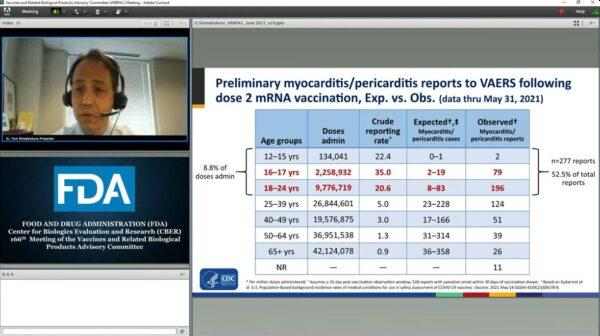
What’s even more disturbing about the report is that “teenagers and people in their early 20s accounted for more than half of the myocarditis cases reported to the CDC’s safety monitoring systems following Covid-19 vaccination, despite representing a fraction of people who have received the shots.”
During a presentation to a Food and Drug Administration advisory group, Dr. Tom Shimabukuro, deputy director of the CDC’s Immunization Safety Office, said that overall, 226 cases of myocarditis or pericarditis after vaccination in people younger than age 30 have been confirmed. He said that further investigation is needed. However, Dr. Shimabukuro also added that “We clearly have an imbalance there.”
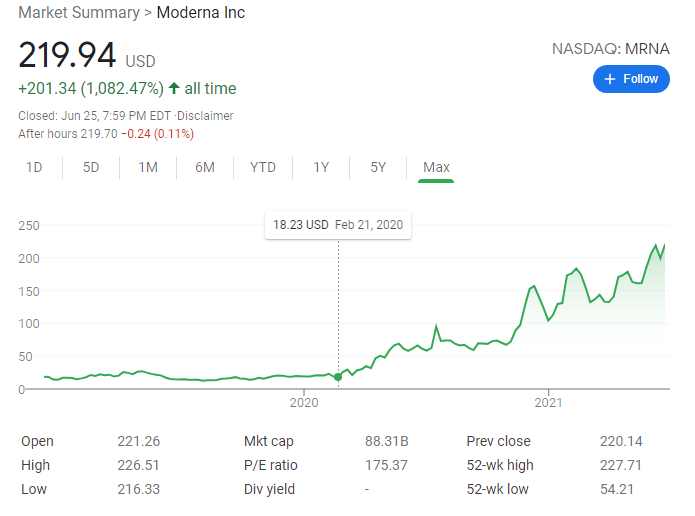
Meanwhile, the pharmaceutical companies continue to rake in billions of dollars even as the FDA the Food and Drug Administration (FDA) expanded its emergency use authorization for the Pfizer vaccine to include children ages 12 to 16. For example, the stock price of Moderna has gone through the roof from a little of $18 in February 2020 to over $200 as of the close of the market last Friday.
Below is the entire press release from the FDA.
The U.S. Food and Drug Administration (FDA) continued to take action in the ongoing response to the COVID-19 pandemic:
- Today, the FDA is announcing revisions to the patient and provider fact sheets for the Moderna and Pfizer-BioNTech COVID-19 vaccines regarding the suggested increased risks of myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the tissue surrounding the heart) following vaccination. For each vaccine, the Fact Sheet for Healthcare Providers Administering Vaccine (Vaccination Providers) has been revised to include a warning about myocarditis and pericarditis and the Fact Sheet for Recipients and Caregivers has been revised to include information about myocarditis and pericarditis. This update follows an extensive review of information and the discussion by CDC’s Advisory Committee on Immunization Practices meeting on Wednesday. The data presented at this meeting reinforced the FDA’s decision to revise the fact sheets and further informed the specific revisions. The warning in the Fact Sheets for Healthcare Providers Administering Vaccines notes that reports of adverse events suggest increased risks of myocarditis and pericarditis, particularly following the second dose and with onset of symptoms within a few days after vaccination. Additionally, the Fact Sheets for Recipients and Caregivers for these vaccines note that vaccine recipients should seek medical attention right away if they have chest pain, shortness of breath, or feelings of having a fast-beating, fluttering, or pounding heart after vaccination. The FDA and CDC are monitoring the reports, collecting more information, and will follow-up to assess longer-term outcomes over several months.
- Today, the FDA issued a supplement to the 2015 safety communication on reprocessed flexible bronchoscopes, reminding health care facilities to follow manufacturer instructions for reprocessing and device maintenance, and providing a new recommendation for health care providers on single-use bronchoscopes. The goal of the recommendations is to reduce potential infection transmission between patients. This communication also includes updated information on medical device adverse event reports.
- The FDA reached a milestone of approving 1,000 original and supplemental generic drug applications to help in the treatment of patients with COVID-19 since the start of the pandemic. The Center for Drug Evaluation and Research prioritized the review of generic drug applications for potential treatments and supportive therapies for patients with COVID-19, such as antibiotics, sedatives used in ventilated patients, anticoagulants, and pulmonary medications. Reaching this milestone during the COVID-19 pandemic further supports the FDA’s everyday mission of improving access to safe, high-quality treatment options, which can result in more competition in the market and more affordable medicines for Americans.
- As part of the FDA’s effort to protect consumers, on June 22, 2021, the agency issued a warning letter to Pacific Center of Health/Pacific Center of Health & Acupuncture for selling unapproved products with unproven COVID-19 claims. Consumers concerned about COVID-19 should consult with their health care provider.
- Testing updates:
- As of today, 389 tests and sample collection devices are authorized by the FDA under emergency use authorizations (EUAs). These include 278 molecular tests and sample collection devices, 83 antibody and other immune response tests and 28 antigen tests. There are 52 molecular authorizations and one antibody authorization that can be used with home-collected samples. There is one molecular prescription at-home test, three antigen prescription at-home tests, five antigen over-the-counter (OTC) at-home tests and two molecular OTC at-home tests.
- The FDA has authorized 11 antigen tests and three molecular tests for serial screening programs. The FDA has also authorized 544 revisions to EUA authorizations.