Scientists reverse diabetes in mice by hacking human cells to make insulin
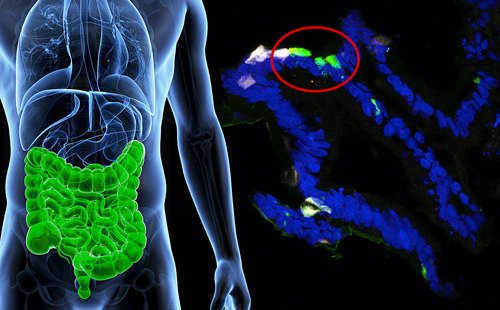
In an exciting breakthrough, scientists from Weill Cornell Medicine in New York have successfully repurposed human stomach cells, turning them into insulin-releasing tissues that respond to elevated blood sugar levels. This discovery holds great potential for enabling individuals, especially those with type 1 diabetes, to better manage their condition in a more organic manner.
The process for creating the tissues isn’t particularly complicated and the tissues can last for many months after being transplanted. There are several differences between human and mouse stomach tissue that need to be addressed in future studies, but initial signs are promising.
In research published in Nature Cell Biology, the researchers explained that the process of creating the tissues isn’t particularly complicated and the tissues can last for many months after being transplanted. There are several differences between human and mouse stomach tissue that need to be addressed in future studies, but initial signs are promising.
The experiment was led by researchers from Weill Cornell Medicine in the United States. They revealed the transplants of gastric insulin-secreting (GINS) cells reversed diabetes in mice.
Under normal circumstances, the responsibility of releasing the hormone insulin in response to high sugar levels in the bloodstream falls upon pancreatic beta cells. However, individuals with diabetes suffer from damaged or depleted beta cells, hindering their ability to effectively transport glucose into cells for energy.
Although gastric insulin-secreting (GINS) cells are not identical to beta cells, they possess the ability to imitate their functionality. The gastrointestinal tract harbors numerous stem cells, which have the remarkable capacity to differentiate into various cell types and undergo rapid multiplication. The prospect arises that individuals with diabetes could potentially undergo a transformation of their own gut stem cells into GINS cells, thereby minimizing the risk of rejection and offering a promising solution.
“The stomach makes its own hormone-secreting cells, and stomach cells and pancreatic cells are adjacent in the embryonic stage of development, so in that sense it isn’t completely surprising that gastric stem cells can be so readily transformed into beta-like insulin-secreting cells,” says Joe Zhou, an associate professor of regenerative medicine at Weill Cornell Medicine in New York.
Scientists have dedicated numerous years to achieving a breakthrough like this but with limited success. In this particular study, the research team focused on activating three specific proteins in a sequential manner within the cells responsible for gene expression. This activation sequence triggered a transformation of these cells into gastric insulin-secreting (GINS) cells.
The reprogramming process proved to be remarkably efficient. When these cells were grown in organoids, small clusters of cells, they demonstrated a heightened sensitivity to glucose. Furthermore, the team observed long-lasting effects on diabetes in mice after the transplantation of these GINS organoids.
According to the researchers, the process of producing GINS cells from stomach cells is relatively uncomplicated. It merely takes a few days, and these newly formed organoids exhibit durability for several months following transplantation, as evidenced by their experiments.
In their report, the researchers state, “Gastric insulin-secreting (GINS) organoids exhibited glucose responsiveness 10 days after induction. They were stable upon transplantation for as long as we tracked them (6 months), secreted human insulin, and reversed diabetes in mice.”
The insulin hormone plays a crucial role in regulating blood glucose levels, and insufficient levels can lead to various health complications. Diabetes affects millions of individuals worldwide, with many relying on insulin injections to manage their glucose levels effectively.
While this approach is still in its early stages, it holds the potential to restore a more natural regulation of insulin levels within the body. The researchers acknowledge several variances between human and mouse stomach tissue that require further investigation in future studies. Additionally, they aim to enhance the resilience of GINS cells against attacks from the immune system.
Nevertheless, the initial findings are promising. This research contributes to the growing body of knowledge on combating diabetes, which includes dietary improvements and refining insulin delivery methods.
Zhou, one of the researchers, remarks, “This is a proof-of-concept study that gives us a solid foundation for developing a treatment, based on patient’s own cells, for type 1 diabetes and severe type 2 diabetes.”