FDA rejects Elon Musk’s Neuralink application to test brain chips in humans, citing safety risks

The U.S. Food and Drug Administration (FDA) has rejected Neuralink’s application to test brain chips in humans, Reuters reported, citing seven current and former employees.
According to the report, Musk has predicted on at least four occasions since 2019 that his medical device company, Neuralink, would soon start human trials of the wireless brain implant. Musk said the revolutionary implant will be able to cure a series of neurological disorders including memory loss, hearing loss, blindness paralysis, depression, insomnia, seizures, addiction, brain damage, and strokes, among others.
The application rejection has not been previously reported. In explaining the decision to Neuralink, the FDA “outlined dozens of issues the company must address before human testing, a critical milestone on the path to final product approval,” the staffers said.
The employees told Reuters that FDA’s major safety concerns “involved the device’s lithium battery; the potential for the implant’s tiny wires to migrate to other areas of the brain; and questions over whether and how the device can be removed without damaging brain tissue.”
According to CNBC, industry players who have been closely watching Neuralink’s development have long expected a collision between Musk and the FDA. For example, Kip Ludwig, former program director for neural engineering at the U.S. National Institutes of Health (NIH), said:
“Everybody in the industry was saying: ‘Oh my God, they’re going to run straight into a brick wall,’” Ludwig said of Musk’s bid for FDA approval. “Neuralink doesn’t appear to have the mindset and experience that’s needed to get this to market anytime soon.”
As you may recall in the summer of 2020, Elon Musk demonstrated Neuralink’s AI-powered brain implant in live pigs. During the demonstration, Musk showed the uses and applications of Neuralink with three pigs implanted with Link V0.9 chips. Musk told the audience that the implementation would one day be able to cure affectations of the human body such as blindness, and brain damage, and cure addictions.
The Neuralink device is called Link V0.9. It is a very small device and just 23 x 8 mm and is implanted directly in the upper cranial cortex, with surgery that, according to Musk, can be performed in less than an hour and without the need for general anesthesia.
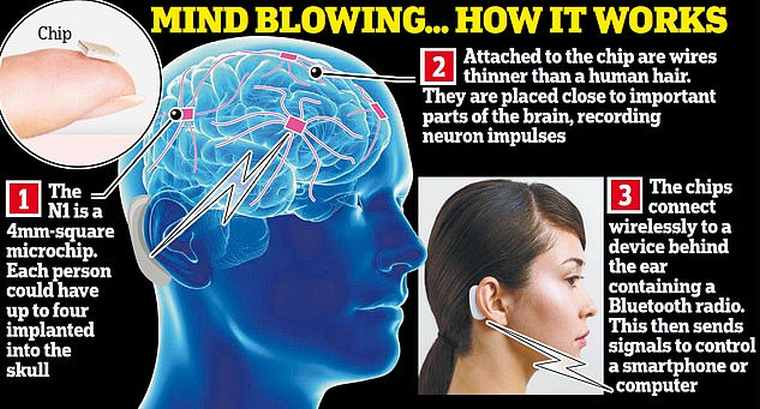
The Link V0.9 device has a multitude of sensors, temperature, pressure, six-axis accelerometer, and promises to charge for the whole day, with charging by induction when necessary. Its main function is to record and transmit information in real-time from brain neurons to a computer, with its 1,024 electrodes (channels), for its interpretation and study.
Co-founded by Musk in 2016, the San Francisco-based Neuralink is working towards improving the brain-machine implant process until the procedure becomes as seamless as Lasik. The Neuralink device can make anyone superhuman by connecting their brains to a computer.

