South Korea-based biotech startup LISCure Biosciences secures $21M in funding to develop immunotherapy drugs to help the body immune system fight cancer

The use of immunotherapy treatments for cancer has been gaining momentum in recent years as government researchers and drug makers work together to find a lasting cure for cancer and other diseases. Unlike traditional drugs, immunotherapy is a type of cancer treatment that helps your immune system fight cancer. Immunotherapy uses substances to stimulate or suppress the immune system to help the body fight cancer, infection, and other diseases.
One of the new startups in this space is LISCure Biosciences, a Seoul, South Korea-based biotech startup that researches and develops ‘bacteria-mediated immunotherapy’ that can treat people by itself or be used in combination with other drugs for major indications such as tumors, degenerative neurological diseases, autoimmune diseases, metabolic diseases.
Today, LISCure announced that it has closed a $21 million Series B funding round to further develop its pipeline and enhance its R&D capabilities of key technologies. Backers include institutional investors, venture capitals, and KOSDAQ listed companies (as strategic investors).
LISCure uses a single strain approach whose strain is a naturally derived as well as non-pathogenic substance so it has a great advantage over other microbiome competitors in terms of safety. LISCure is developing the world’s first microbiome-based NASH(non-alcoholic steatohepatitis) treatment, LB-P8, as well as the rheumatoid arthritis treatment, LB-P6, for global clinical trials.
Founded in 2018, LISCure Biosciences is a developer of bacteria-mediated immunotherapy designed to treat tumors as well as degenerative neurological, autoimmune, and metabolic diseases. The company currently has seven pipelines of medicine and two pipelines of health-functioning food and is considering the introduction of two more pipes.
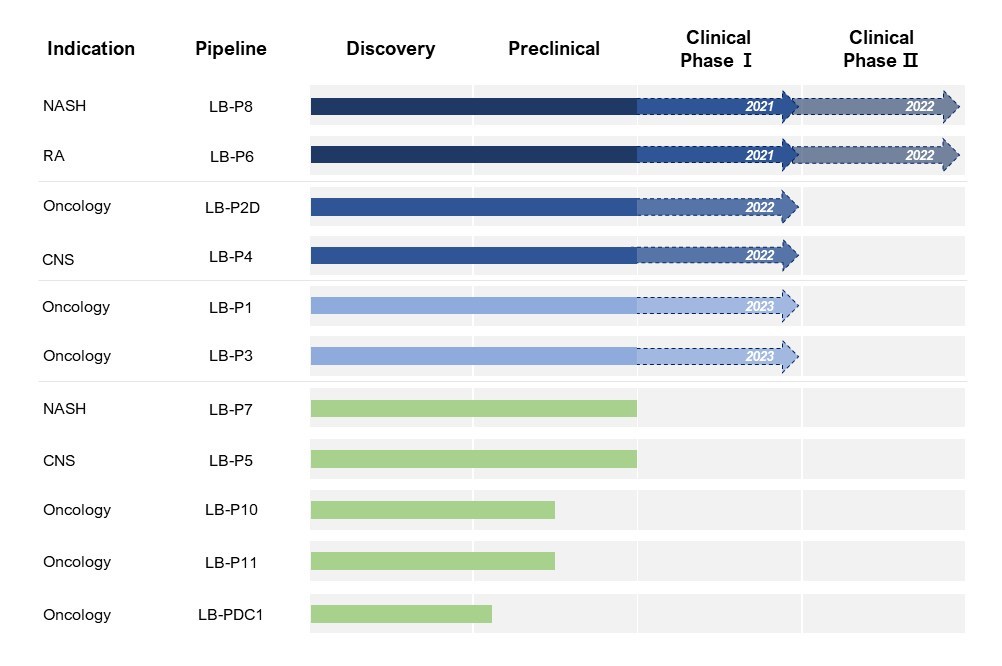
LISCure Biosciences’s pipeline
“This investment was made after reviewing the promising pre-clinical results of LISCure’s new drug candidates for each indication and we verified its potential as a new therapeutic treatment. Based on the technologies of both companies, we will actively cooperate in research and development for these innovative new drug candidates in the microbiome field”, one of the strategic investors said.
LISCure’s drug candidates have already been tested for efficacy and safety by a third CRO, and four of the candidates have been completed the process development by CDMO based in the United States. Two of the candidates are currently in the process of cGMP manufacturing under CDMO based in France. LISCure is expecting the four lead candidates to enter global clinical trials in the next two years and possibly begin phase 2 with two of the candidates.
In addition to the funding, LISCure has completed the establishment of corporations in the US and Australia for global clinical development. LISCure is planning to expand indications of each program through the US subsidy, working with global top research institutes.

