Gilead says 7% of COVID-19 patients on Remdesivir developed acute kidney injury. Gilead also says in its clinical data that “It’s NOT yet known if Remdesivir is safe and effective for the treatment of COVID-19”
We’ve been following Gilead’s remdesivir (sold under drug name Veklury) for over five months now. In April, Remdesivir’s study was touted by Dr. Anthony Fauci as a smashing success. Gilead’s stock also soared after the news. A lot has changed since we last wrote about the drug and many experts are wondering if remdesivir really saves lives.
In the latest clinical data provided on its website, Gilead says 5% of COVID-19 patients reported serious adverse events (SAEs) with remdesivir, 7% of patients developed acute kidney injury, 8% of patients developed anemia or decreased hemoglobin, and 4% of the patients discontinued the use of the drug due to adverse events. Gilead also said that “it is not yet known if Veklury [remdesivir] is safe and effective for the treatment of COVID-19.”
On May 1, 2020, as part of its effort to help COVID-19 patients, the Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) for emergency use of Veklury (remdesivir) for the treatment of hospitalized patients with severe 2019 coronavirus disease (COVID-19). Veklury is a direct-acting antiviral drug that inhibits viral RNA synthesis.
However, in a piece published in FiercePharma, an online publication for the pharmaceutical industry, FiercePharma author Eric Sagonowsky said, “Skeptics question Gilead data showing remdesivir cut death rates.” Below is how FiercePharma explains it.
Gilead Sciences released new data Friday showing its antiviral remdesivir slashed death rates by 62% compared with standard treatment, but health experts aren’t convinced. Gilead compared 312 patients dosed with remdesivir in its phase 3 SIMPLE trial against a historical cohort of 818 patients with similar disease severity on “standard of care,” but because that comparison wasn’t performed in a controlled trial, the numbers are “deeply flawed,” one expert said. Gilead itself said the findings “[require] confirmation in prospective clinical trials.”
Then just three days ago, on August 28, 2020, the FDA broadens Emergency Use Authorization for Veklury (remdesivir) to include all hospitalized patients for treatment of COVID-19. However, as USA Today puts it, ‘Without evidence’: Once again, FDA expands use of COVID-19 treatment without research to back it up.”
“It seems to be a pattern of approval without science, without data, without evidence,” said Dr. Eric Topol, vice president for research at Scripps Research in La Jolla, California, and a national expert on the use of data in medical research. Topol said he was appalled by the expanded approval of the drug. “There are no data to support wide use of remdesivir,” he said. “This is extraordinary.”
So, what’s really going on with remdesivir? Rather than making up stories and providing misleading information public about drug, we think the best way is to let the drugmaker, Gilead, tell you about the drug itself. Surprisingly, Gilead recently updated its website where it provides detailed clinical data about remdesivir.
Here’s what Gilead says on the Clinical Data page of the website. The information provided below is as of August 31, 2020.
Remdesivir (VEKLURY) is an investigational drug that has not been approved by the FDA for any use. It is not yet known if VEKLURY is safe and effective for the treatment of COVID-19.
In a bold print, Gilead added saying remdesivir is an unapproved antiviral drug and the risks and benefits of the use of VEKLURY for the treatment of COVID-19 are not yet known.
VEKLURY is an unapproved antiviral drug with available data from 2 randomized clinical trials in patients with COVID-19. The risks and benefits of the use of VEKLURY for the treatment of COVID-19 are not yet known.
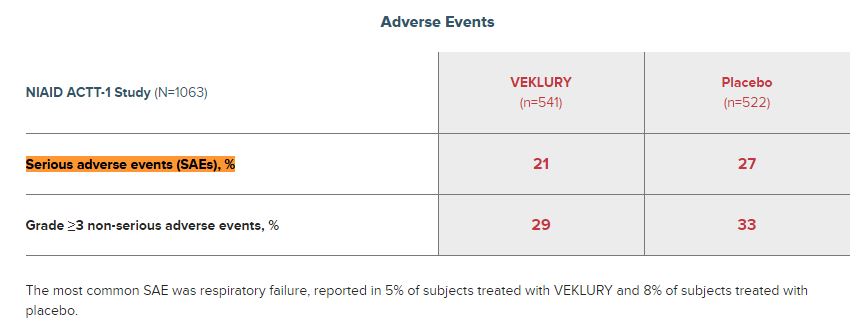
As you go down to the “Adverse Events” section of the Clinical Data page, Gilead says 5% of COVID-19 patients reported serious adverse events (SAEs) versus 8% of patients treated with placebo.
The most common SAE was respiratory failure, reported in 5% of subjects treated with VEKLURY and 8% of subjects treated with placebo.
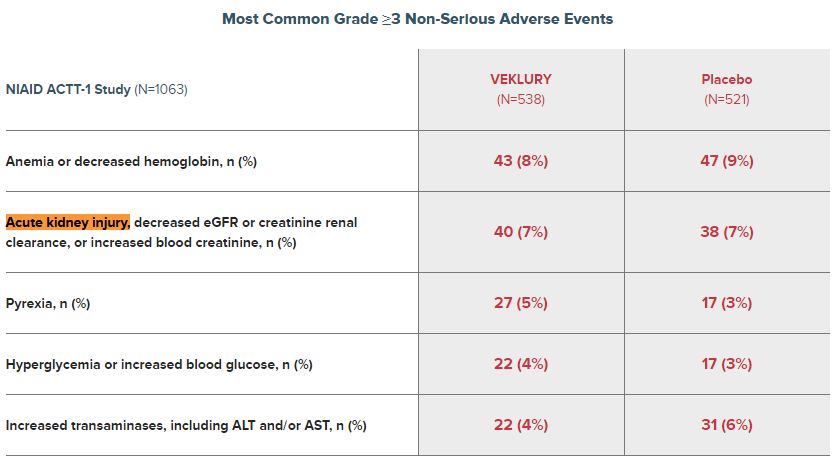
Gilead also provided data on non-serious adverse events: 8% of patients developed anemia or decreased hemoglobin, 7% of patients developed acute kidney injury, decreased eGFR or creatinine renal clearance, or increased blood creatinine, and 4% developed increased transaminases, including ALT and/or AST.
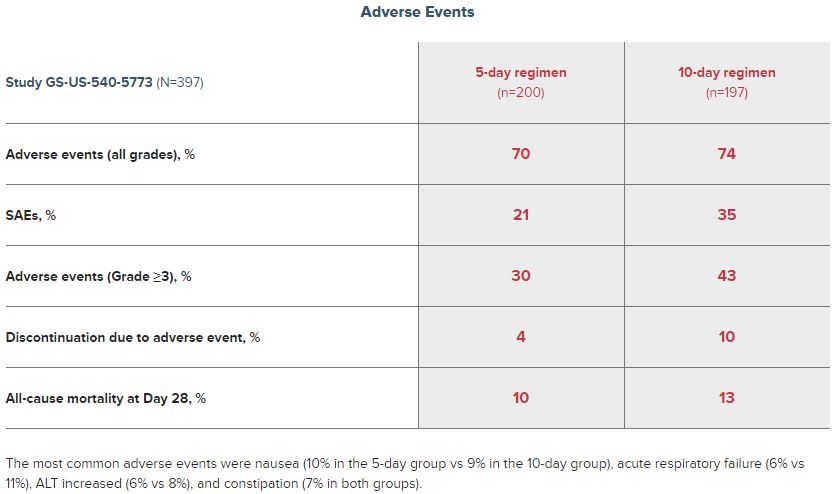
Then, Gilead also has a section for just adverse effects. Gilead says the most common adverse events were nausea (10% in the 5-day group vs 9% in the 10-day group), acute respiratory failure (6% vs 11%), ALT increased (6% vs 8%), and constipation (7% in both groups). According to Gilead, 4% of the patients discontinued the use of remdesivir due to adverse events.
Gilead concludes with remdesivir’s safety information. Below is what Gilead says:
Safety Information
VEKLURY is an unapproved investigational product, and there are limited clinical data available. Serious and unexpected adverse events may occur that have not been previously reported with VEKLURY use.
Rather than reaching our own conclusion, we recommend you take a hard look at the clinical data provided by Gilead and draw your own conclusion.

