Chicago-based Healthtech startup physIQ is using its Ebola expertise and new machine learning analytics to fight and detect early signs of COVID-19
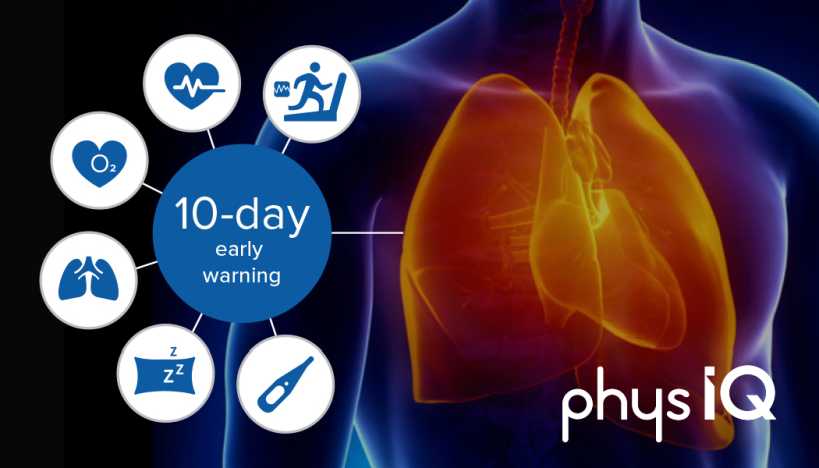
As we all know, early diagnosis of disease generally increases the chances for successful treatment by focusing on detecting symptomatic patients as early as possible. The same can be said of coronavirus or other infectious diseases–early detection is the critical to successful treatment. That’s the reason why this Chicago-based startup, physIQ, is using over one million hours of training set data to implement new machine learning analytics aimed at detecting early signs of COVID-19. Having previously supported the fight against Ebola in Sierra Leone, Africa, physIQ is now using its expertise to help mitigate a surge of hospital patients and reduce patient and healthcare provider exposures during the COVID-19 pandemic.
Founded in 2013 by Gary Conkright and Matt Pipke, physIQ has developed a personalized physiology analytic platform (PPA) for use in both the regulated healthcare market as well as the unregulated health and fitness market. The technology is designed to detect subtle changes in vital signs and other health indicators to help clinicians manage patients with chronic illness, and to help consumers.
Since the coronavirus outbreak here in the United States, physIQ is in the frontline of health innovation. Promptly following the Food and Drug Administration’s (FDA) sanctioned labeling of pinpointIQTM and its multivariate change index (MCI) to allow for remote monitoring of patients with COVID-19, physIQ released its set of clinical “rules” representative of clinical deterioration in patients diagnosed with or exposed to COVID-19.
With this extensive experience monitoring Ebola patients, in combination with the over one million hours of annotated physiologic data, physIQ has provided a unique perspective on how to develop this new set of rules. physIQ’s analytics leverage their large trove of physiologic data, that was collected in partnership with the life-science industry and Veterans Affairs Administration (VA), and uses iterative processing and artificial intelligence modeling to learn the baseline physiologic patterns of individual patients under normal conditions. From that “normal” baseline, the analytics continuously monitor for statistical changes, suggestive of clinical deterioration.
Its PinpointIQTM platform can deliver on two additional principles that are critical to management of COVID-19. First, remote monitoring may ease the burden on hospitals and other healthcare facilities by reducing the risk of exposure for patients and healthcare professionals to SARS-CoV-2 (the virus that causes COVID-19) and to conserve resources for the very sickest. Secondly, early detection of clinical deterioration associated with COVID-19 may promote better outcomes. The potential utility of pinpointIQTM used with COVID-19 patients is corroborated by the work physIQ has done with USAID in patients with Ebola, as well as in clinical studies conducted with the VA in patients with severe congestive heart failure.
“This capability does not happen overnight, and not something that can be developed in a few days or few weeks if done correctly,” said Stephan Wegerich, physIQ’s Chief Science Officer. “We’ve learned what it takes to effectively apply analytics from the hundreds of thousands of hours of data we’ve collected from chronically ill patients and patients undergoing treatment for a variety of illnesses. This is not something we just started working on since COVID-19 came to the United States.”
“Our entire healthcare infrastructure is strained at best by COVID-19,” noted Gary Conkright, CEO of physIQ. “In many cases, we will have more patients than hospital beds and our only option is to find ways to better care for patients at home. With clinical grade wearable sensors and our proprietary, FDA-cleared analytics, we are providing hospitals with personalized physiologic visibility into their homebound high-risk COVID-19 patients. We believe this will not only free up hospital capacity, but also reduce clinician exposure to this highly virulent disease.”
PhysIQ is pursuing multiple clinical use cases in parallel including COVID-19, chronic diseases such as heart failure or COPD, and responses to chemotherapy and other immune-suppressive therapy. The system is prescribed by a physician and is shipped directly to patient’s home without requiring direct physical contact from a clinician.

