Clinical trials for COVID-19 are expected to complete by the end of April
There is more good news this afternoon that the results from clinical trials for COVID-19 could start to roll in any day, with antimalarials coming through first, according to a report of BioCentury’s analysis of study completion dates in public databases. Based on the data obtained from ClinicalTrials.gov and ChiCTR.org.cn, clinical trials for COVID-19 are expected to complete by April 2020.
Timeline includes trials in Phase II-IV and other efficacy studies without a phase listed. The dates on the timeline are primary endpoint completion dates where available, otherwise the final trial end date was used. Not included are trials primarily testing traditional Chinese medicines or patient plasma. According to the report, almost 30 efficacy trials of repurposed agents for COVID-19 have completion dates that fall on or before April 30.
As we reported yesterday about the new academic study which revealed that over-the-counter anti-malaria pill Chloroquine may be highly effective at treating coronavirus, seven of the these clinical testing are antimalarial agents, four of which are at the front end of the pipeline. Two trials testing the approved antimalarial agent hydroxychloroquine were due to complete Feb. 29 and two others testing chloroquine phosphate are expected to finish March 17, according to ChiCTR.org.cn.
Antivirals make up the largest group, with eight trials due to complete by the end of April. Among them is remdesivir from Gilead Sciences Inc., which WHO has prioritized (see “Preclinical Potencies of Clinical COVID-19 Candidates”).
The first two of the five trials testing remdesivir have completion dates in early April. Both are Phase III trials being run by Chinese groups. Unlike Gilead’s two Phase III studies, both of the China studies are blinded and placebo-controlled, and therefore could give a better read on efficacy (see “NIH, Hubei Trials Could be More Telling than Gilead’s”).
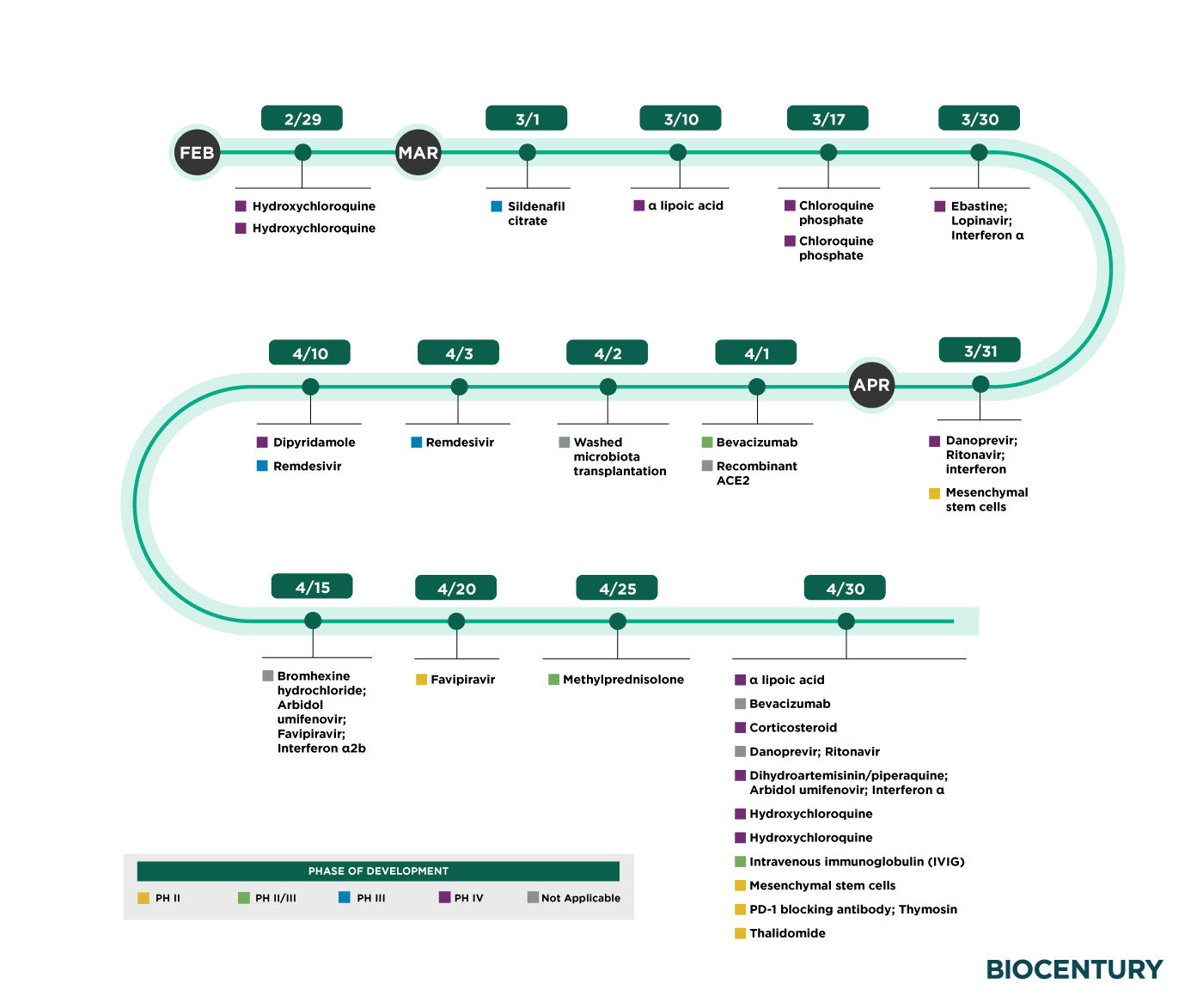
Source: BioCentury
COVID-19 trial timelines