Tokyo tech startup Heartseed secures $26M Series B to accelerate development of iPSC-derived regenerative medicine for heart failure
Cardiovascular disease is the number one killer worldwide. Enter Heartseed, a developing PSC-derived cardiomyocytes for the treatment of heart failure. Heartseed is changing the world using regenerative medicine. Its breakthrough technology will open the door of intractable disease treatment with regenerative medicines.
Today, Heartseed announced it has raised 26 Million (2.8 Billion-yen) Series B funding to accelerate development of iPSC-derived regenerative medicine for heart failure. The round, which brings its total funding to $35 million (3.8 Billion yen) since inception in 2015, was led by new investors, SBI Investment, JMDC, Gene Techno Science, Nissay Capital, SMBC Capital, and an existing investor, Astellas Venture Management LLC., which has supported Heartseed from Series A.
Heartseed was founded in 2015 to develop and commercialize cardiac regenerative medicine developed by Prof. Keiichi Fukuda and his group at the Department of Cardiology, Keio University, Tokyo. Heartseed has original technologies throughout the process of manufacturing and delivering iPSC-derived cardiac regenerative medicine, including purification, cell delivery and iPSC production.
“I have been involved in the research of cardiac regenerative medicine for the past 20-years as a pioneer in this field and solved all the major challenges such as ventricular-specific cardiomyocyte differentiation, purification, large-scale manufacturing and efficient cell delivery,” said Prof. Keiichi Fukuda, co-founder and CEO at Heartseed, Professor at the Department of Cardiology, Keio University, Tokyo. “We are grateful for the support of our investors, which I believe is a reflection of their expectation and confidence that our lead pipeline HS-001 can be a curative therapy for severe HF, with the mechanism that transplanted ventricular-specific highly-purified cardiomyocytes engraft to patient’s heart and retain for a long-term.”
The company expects to initiate Phase 1/2 clinical trial for its lead pipeline HS-001 for HF with reduced Ejection Fraction (HFrEF) in late 2020. The company is also supporting an investigator-initiated clinical trial for Dilated Cardiomyopathy led by Keio University, which is expected to be initiated in the first half of 2020.
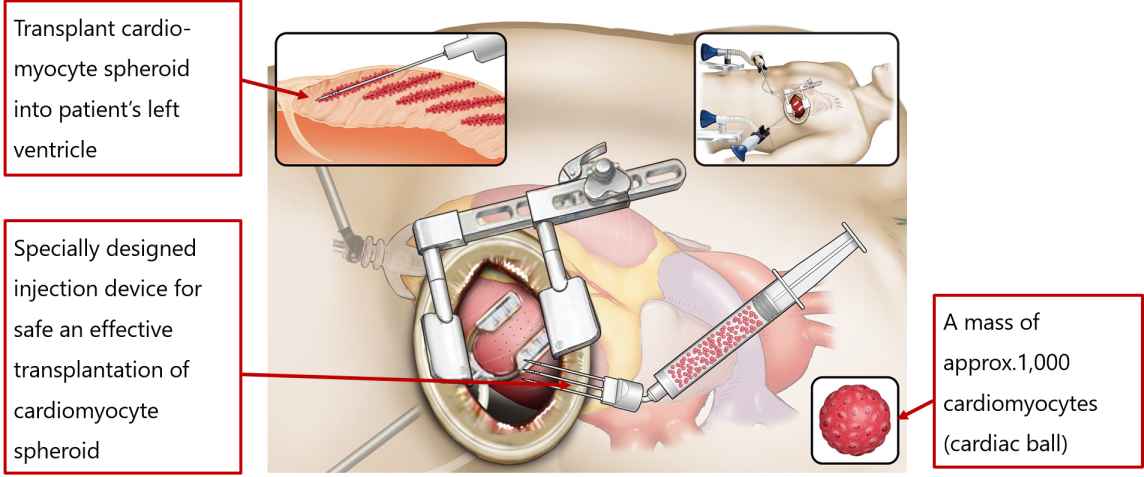
iPSC-derived regenerative medicine for heart failure by Heartseed

