Silicon Valley startup Auris Health receives FDA clearance for its surgical robotics designed to treat lung cancer
Auris Health, Inc. (“Auris Health”), formerly known as Auris Surgical Robotics, is developer of robotic microsurgical devices designed for ophthalmic procedures. The company is engaged in the development of surgical robotics and visualization technology for surgical applications that require very fine movements, improving healthcare for all patients who need medical intervention. As we reported this morning in our top tech startup news stories, Auris Health has quietly raised $500 million to help diagnose cancer using controllers. The company is backed by leading technology investors including Mithril Capital Management, Lux Capital, Coatue Management, and Highland Capital.
In a press release, the company announced yesterday that it has received a U.S. Food and Drug Administration (FDA) clearance for its Monarch™ Platform. The FDA approval ushers in a new era of medical intervention. Founded in 2007 and led by surgical robotics pioneer Frederic Moll, M.D., Auris has raised more than $500 million in equity capital from leading technology investors including Mithril Capital Management, Lux Capital, Coatue Management, and Highland Capital. Auris Health develops robotics technology for medical applications. The company is pioneering the next era of medical intervention by developing platforms that enhance physician capabilities, evolve minimally invasive techniques, and create new categories of care that redefine optimal patient outcomes. The company is committed to transforming medical intervention by integrating robotics, micro-instrumentation, endoscope design, sensing, and data science into one platform. Every element of our technology is driven by patient-specific design aimed at maintaining the integrity of the human body.
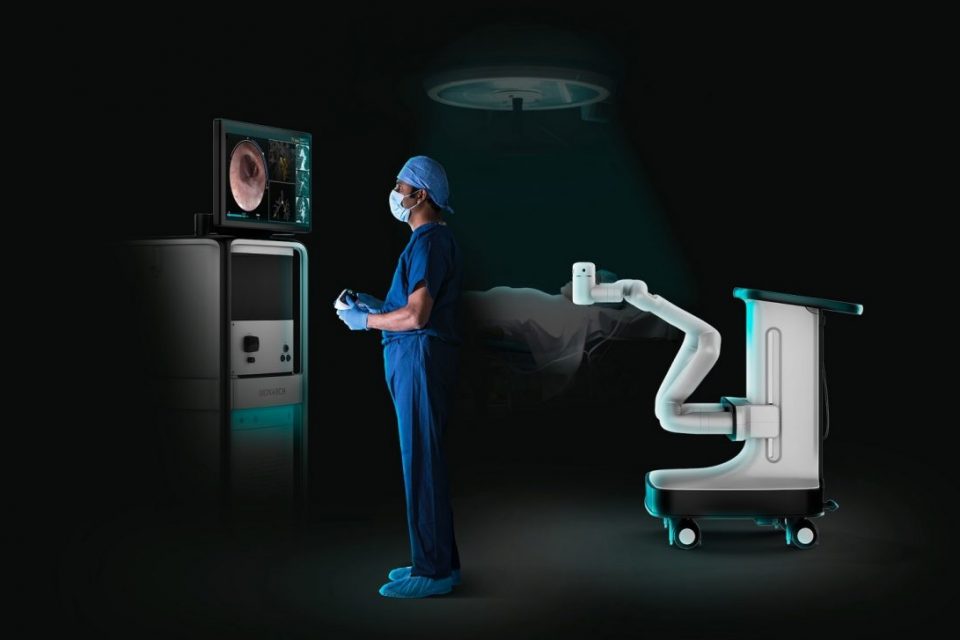
With the introduction of the Monarch Platform, Auris seeks to transform endoscopy, the use of small cameras and tools to enter the body through its natural openings. The Monarch Platform integrates the latest advancements in robotics, software, data science, and endoscope innovation, with the goal of dramatically improving patient outcomes, enhancing physician capabilities, and lowering costs to the healthcare system.
Auris’ first targeted disease state is lung cancer. The FDA cleared the platform for diagnostic and therapeutic bronchoscopic procedures. The goal of the technology is to enable more-accurate diagnosis, and eventually treatment, of small and hard-to-reach nodules in the periphery of the lung. “Technology has advanced significantly since the development of the earliest robotics platforms used in medicine,” said Dr. Moll, CEO, Auris Health. “The Monarch Platform is designed to address the limitations of current technology with the introduction of a new era of flexible robotics. With this FDA clearance, we intend to deliver on the promise of improving patient care, starting with earlier and more accurate diagnosis of pulmonary nodules. We envision additional uses for the technology across future endoscopic clinical indications.”
Lung cancer is the leading cause of cancer deaths worldwide. More patients die every year from the disease than from prostate, breast, and colon cancers combined. More than 90 percent of people diagnosed with lung cancer do not survive, in part because it is often found at an advanced stage. There are a variety of diagnostic options currently available for lung cancer, but all have limitations in accuracy, safety, or invasiveness. These limitations can lead to false positives, false negatives, or side effects such as pneumothorax (collapsed lung) and hemorrhage, which may increase health care costs and extend hospital stays.
“Four hundred fifty people die every day in the United States due to lung cancer. It is the number one cancer killer of both men and women in the world. Lung cancer screening has given us an opportunity to save some of these people by diagnosing the cancer early, while we have a chance to cure it. Despite the benefit, we still are limited by the current technology in making a diagnosis,” said Michael Simoff, M.D., Director of Interventional Pulmonology at Henry Ford Health System in Detroit, Mich. “The development of new advanced technology, like the Monarch Platform, could allow us the opportunity to make the diagnosis early, which translates directly to saving lives.”
The Monarch Platform is a revolutionary flexible endoscopic technology that holds promise to fight lung cancer by allowing physicians to diagnose, and eventually treat, hard-to-reach, small peripheral nodules with greater precision than ever before. The Monarch Platform utilizes a familiar controller-like interface that physicians use to navigate the flexible robotic endoscope to the periphery of the lung with improved reach, vision, and control. Combining traditional endoscopic views into the lung with computer-assisted navigation based on 3-D models of the patient’s own lung anatomy, the Monarch Platform provides physicians with continuous bronchoscope vision throughout the entire procedure.