Brain Cancer Cure? Elon Musk reveals WELIREG, the hidden cancer pill that passed Harvard trials and saved his friend’s mom’s life

According to the American Brain Tumor Association, approximately 90,000 people are diagnosed with a primary brain tumor each year, making brain and other central nervous system (CNS) tumors the fifth most common form of cancer. With over 1 million people currently living with a brain tumor diagnosis, the need for effective treatments is more critical than ever. There is now a ray of hope for those suffering from brain cancer, thanks to a recent revelation by Elon Musk.
Musk shared a remarkable story about WELIREG, a drug currently in trials at Harvard University, which reportedly cured his friend’s mother of brain cancer. After doctors told his friend that his mother had only a few months to live, the friend discovered WELIREG during his search for alternative treatment options, leading to her recovery.
Elon Musk Discusses a Hidden Brain Cancer-Curing Drug
In a video posted on X (formerly Twitter), Elon Musk said that WELIREG successfully treated his friend’s mother’s brain cancer after oncologists had told her there was no hope and that she had only four to five months to live.
“There’s a drug that’s in trials, where you take this pill and it actually makes the brain cancer go away, literally.. and it cured his mom,” Musk said.
https://twitter.com/MJTruthUltra/status/1848166871167893749
Potential Breakthrough in Cancer Treatment
What Is WELIREG?
WELIREG (belzutifan) is an FDA-approved oral prescription medicine used to treat adults with von Hippel-Lindau (VHL) disease who need treatment for certain types of tumors, including renal cell carcinoma (RCC), a form of kidney cancer, tumors in the brain and spinal cord known as central nervous system (CNS) hemangioblastomas, and pancreatic neuroendocrine tumors (pNET). These tumors typically don’t require immediate surgery, making WELIREG an important option for managing their progression.
Additionally, WELIREG is prescribed for adults with advanced renal cell carcinoma (RCC) that has spread, particularly in cases where patients have already been treated with PD-1, PD-L1, or VEGF cancer therapies. While it has shown promise in treating these cancers, it’s important to note that WELIREG has not yet been proven safe or effective for use in children.
WELIREG works by inhibiting a protein called hypoxia-inducible factor-2 alpha (HIF-2α), which is involved in cancer cell growth. By targeting this protein, the drug reduces the likelihood of tumor development.
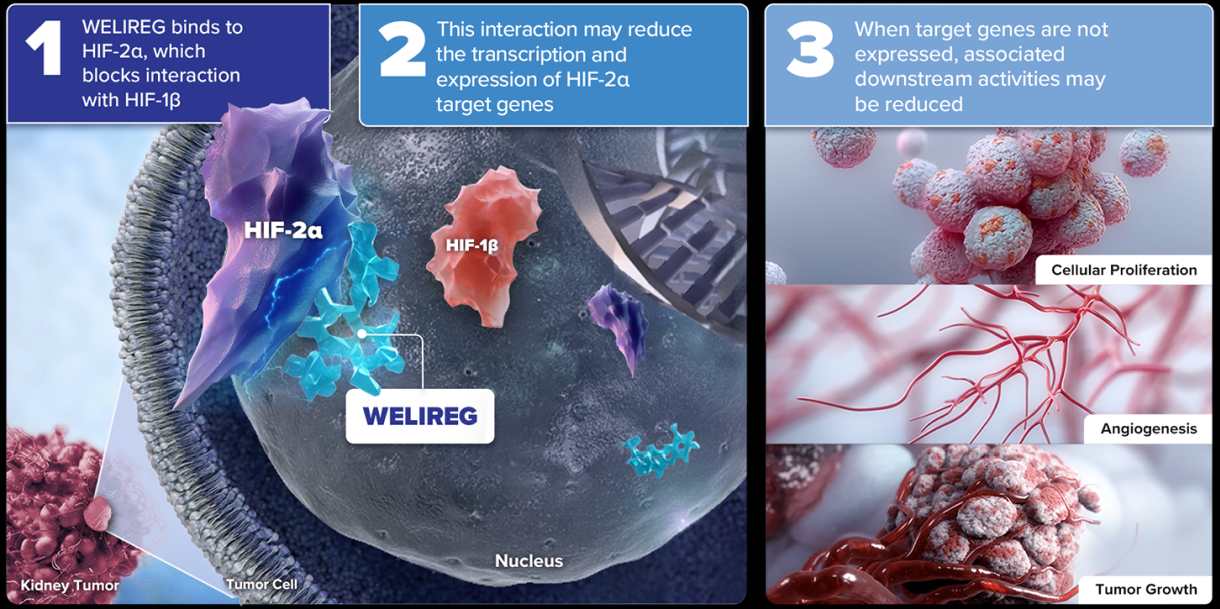
Credit: Welireghcp
Regulatory Hurdles Despite Success in Trials
The drug is currently being tested in trials at Harvard, but Musk noted that regulatory barriers have slowed the process of full approval. The implications of this delay are significant, as WELIREG has already shown the potential to help patients with brain cancer.
Musk’s comments underscore the importance of cutting through red tape to bring this treatment to more people. As he mentioned, his friend’s mother experienced a remarkable recovery thanks to this drug.
How WELIREG Works
WELIREG is a hypoxia-inducible factor-2 alpha (HIF-2α) inhibitor. By blocking the excess production of HIF-2α, which promotes the growth of cancer cells, WELIREG helps prevent the development of tumors. Although still in trials, it holds promise as an innovative approach to cancer treatment.
WELIREG: A New Hope in Brain Cancer Treatment?
Musk’s revelation highlights the potential of WELIREG to become a widely recognized cancer treatment. As discussions about the drug gain momentum, it could pave the way for a new era of brain cancer therapies, especially as more people learn about its trial success.
While challenges remain in getting broader approval, WELIREG’s effectiveness and the story behind it are sparking hope that more patients may benefit from this treatment in the near future.
Important Considerations and Medical Guidance
While Elon Musk highlighted WELIREG for its role in curing his friend’s mother’s brain cancer, it’s important to note that WELIREG is not specifically labeled as a “brain cancer pill.” The drug is approved to treat CNS hemangioblastomas, a type of brain tumor linked to von Hippel-Lindau (VHL) disease. As with any treatment, it’s crucial to consult a healthcare professional to determine the appropriate course of action, understand the drug’s effectiveness, and be aware of any potential side effects when considering it for brain cancer treatment.




