CDC to hold ’emergency meeting” after hundreds suffered rare heart inflammation following Pfizer and Moderna COVID-19 mRNA vaccines

The Centers for Disease Control and Prevention (CDC) announced that that it will convene an “emergency meeting” of its advisers on June 18th to discuss rare but higher-than-expected reports of heart inflammation following doses of the mRNA-based Pfizer and Moderna COVID-19 vaccines, according to news first reported by CBC News.
The emergency meeting is scheduled for June 18, 2021, between 11 AM – 5 PM EST. Some of the items in the meeting agenda include: “Update on COVID-19 vaccine safety, including myocarditis after mRNA vaccines VaST assessment, discussion about benefit and risk of COVID-19 mRNA vaccines in adolescents and young adults, and data to inform recommendations for additional doses of COVID-19 vaccines.
According to CBS News, the CDC has so far identified 226 reports that might meet the agency’s “working case definition” of myocarditis and pericarditis following the shots, the agency disclosed Thursday.
CBS News added that the vast majority have recovered, but 41 had ongoing symptoms, 15 are still hospitalized, and 3 are in the intensive care unit. To date, nearly 130 million Americans have been fully vaccinated with either Pfizer or Moderna’s doses.
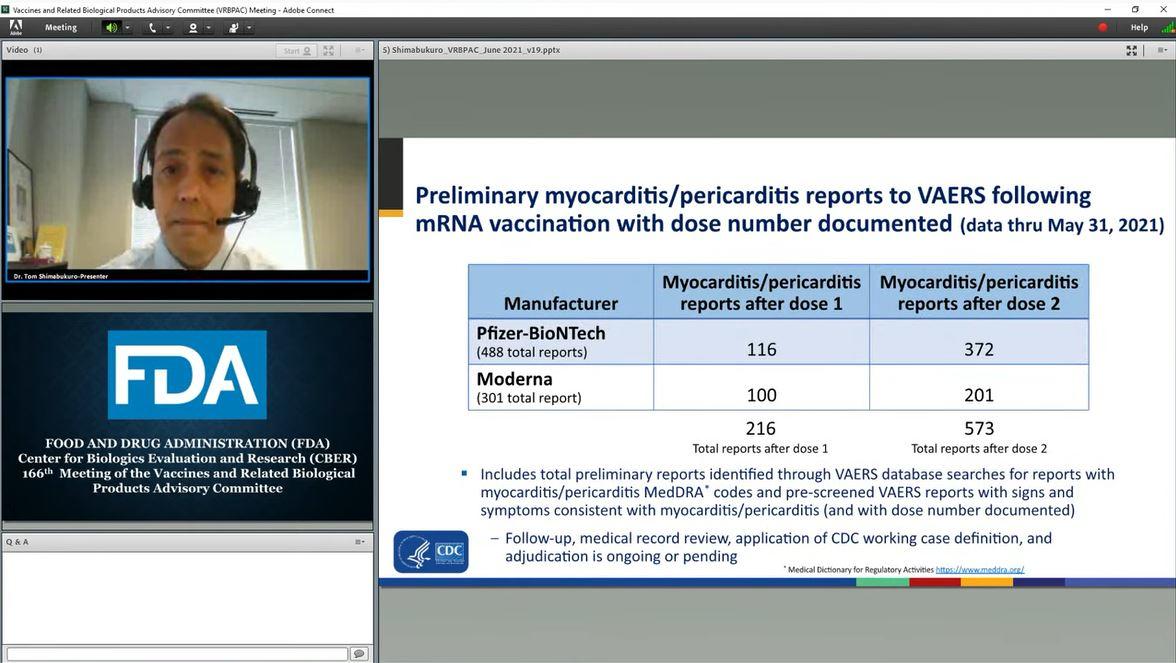
A slide on myocarditis reports post-COVID-19 vaccination is shown during the Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee meeting on June 10, 2021. (FDA/Screenshot via The Epoch Times)
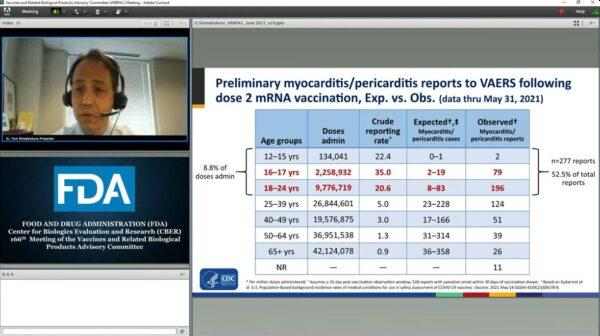
From another slide, Epoch Times wrote: “Of note, of these 528 reports after the second dose with symptom onset within 30 days, over half of them were in these younger age groups, 12–24 years old, whereas roughly 9 percent of total doses administered were in those age groups, so we “clearly have an imbalance there.”
Update: Title changed to reflect additional information about the number of reports. The article was originally titled: “CDC plans ’emergency meeting” on rare heart inflammation following 226 reports from people who took Pfizer and Moderna COVID-19 mRNA vaccines.”




