Coronavirus therapeutic breakthrough: FDA approves emergency use of convalescent plasma treatment for COVID-19 patients
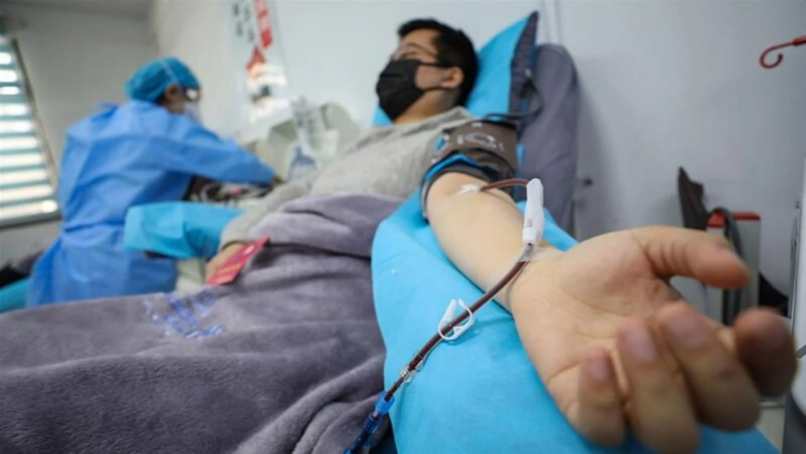
The deadly coronavirus, that started in Wuhan, China in December 2019 has so far claimed over 800,000 lives worldwide with over 180,000 deaths in the US alone. As the race for the vaccines continues, today, the FDA announced the emergency use authorization (EUA) of convalescent plasma treatment for Covid-19 patients. It comes with 35 percent reduction of Covid-19.
The EUA allows the expand the use of convalescent plasma for Covid-19 patients. Over 100,000 Americans have already enrolled in the treatment. U.S. President Trump made the announcement during a news conference Sunday evening.
In another statement on its website, FDA said:
Today, the U.S. Food and Drug Administration issued an emergency use authorization (EUA) for investigational convalescent plasma for the treatment of COVID-19 in hospitalized patients as part of the agency’s ongoing efforts to fight COVID-19. Based on scientific evidence available, the FDA concluded, as outlined in its decision memorandum, this product may be effective in treating COVID-19 and that the known and potential benefits of the product outweigh the known and potential risks of the product.
The EUA authorizes the distribution of COVID-19 convalescent plasma in the U.S. and its administration by health care providers, as appropriate, to treat suspected or laboratory-confirmed COVID-19 in hospitalized patients with COVID-19.
Convalescent plasma has been used throughout history when confronting an infectious disease where you have people who recover and there’s no other therapy available. Convalescent plasma interacts differently with the immune system than a vaccine. When a person is treated with a vaccine, their immune system actively produces its own antibodies that will kill off any future encounters with the target pathogen. That’s called active immunity.
Unlike vaccines, convalescent plasma interacts differently with the immune system than a vaccine. Convalescent plasma offers what’s the medical experts called “passive immunity.” With convalescent plasma, the body doesn’t create its own antibodies, but instead “borrows” them from people who have recovered from the virus.
When a person is treated with a vaccine, for example, their immune system actively produces its own antibodies that will kill off any future encounters with the target pathogen. That’s called active immunity. Unlike a vaccine, the protection doesn’t last a lifetime, but the borrowed antibodies can greatly reduce recovery times and even be the difference-maker between life and death.
Commenting on the development, Health and Human Services Secretary Alex Azar, said:
“The FDA’s emergency authorization for convalescent plasma is a milestone achievement in President Trump’s efforts to save lives from COVID-19,” said Secretary Azar. “The Trump Administration recognized the potential of convalescent plasma early on. Months ago, the FDA, BARDA, and private partners began work on making this product available across the country while continuing to evaluate data through clinical trials. Our work on convalescent plasma has delivered broader access to the product than is available in any other country and reached more than 70,000 American patients so far. We are deeply grateful to Americans who have already donated and encourage individuals who have recovered from COVID-19 to consider donating convalescent plasma.”