BREAKING: Remdesivir only helped those on oxygen. Mortality too high for standalone treatment; new study shows
We’ve been following Gilead’s Remdesivir for over two months now. In April, Remdesivir’s study was touted by Dr. Anthony as a smashing success. The stock market soared after the news. But, it didn’t take more than 24 hours before medical experts started raising questions about the study.
Now, according to a new study published in the New England Journal of Medicine late on Friday, Remdesivir offers no marked benefit for those who were healthier and didn’t need oxygen or those who were sicker, requiring a ventilator or a heart-lung bypass machine.
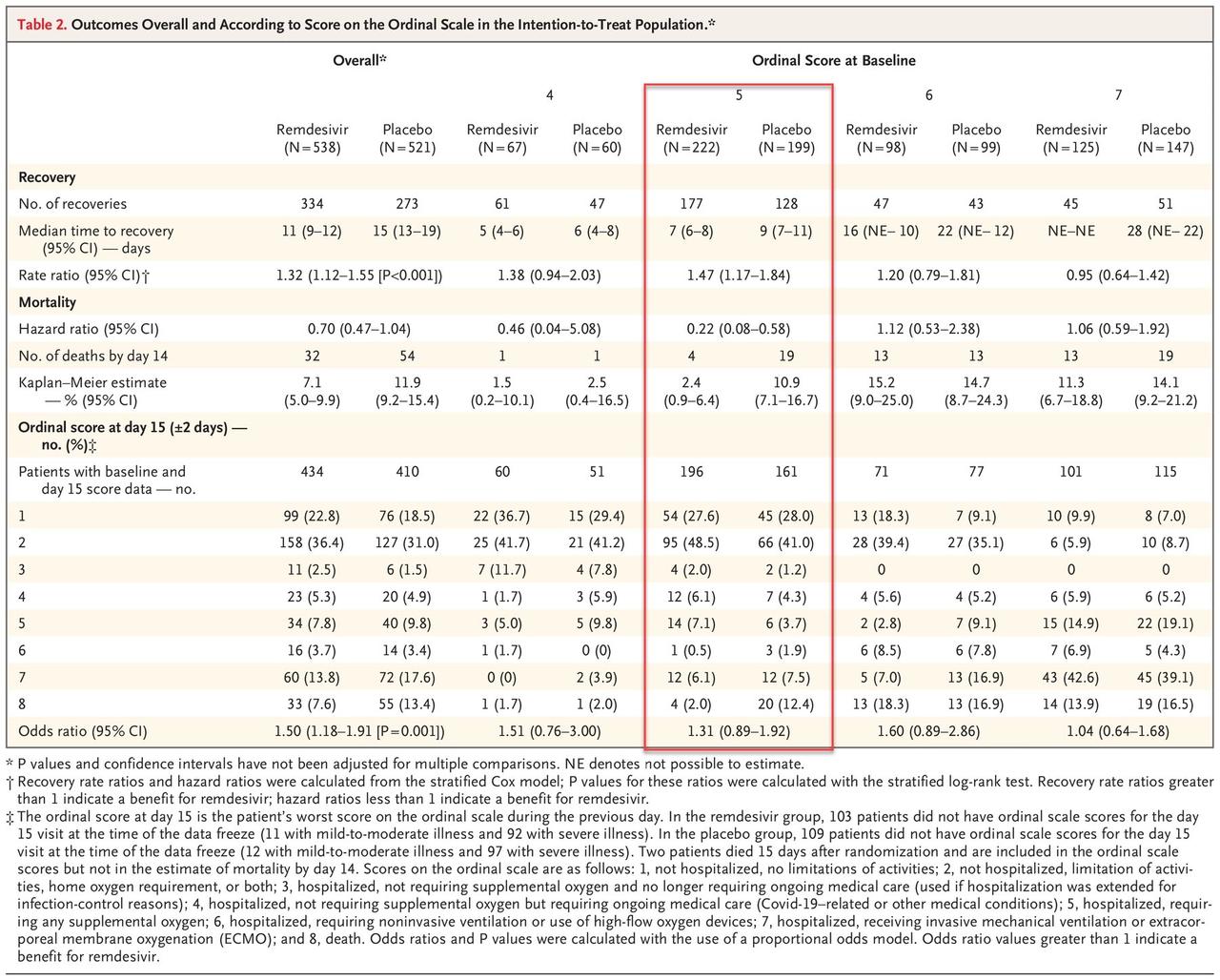
Remdesivir, which was authorized to treat Covid-19 in a group of 1063 adults and children (split into two groups, one receiving placebo instead of remdesivir) who need i) supplemental oxygen, ii) a ventilator or iii) extracorporeal membrane oxygenation (ECMO), only significantly helped those on supplemental oxygen.
As of April 28, 2020, a total of 391 patients in the remdesivir group and 340 in the placebo group had completed the trial through day 29, recovered, or died. Eight patients who received remdesivir and 9 who received placebo terminated their participation in the trial before day 29. There were 132 patients in the remdesivir group and 169 in the placebo group who had not recovered and had not completed the day 29 follow-up visit. The analysis population included 1059 patients for whom we have at least some postbaseline data available (538 in the remdesivir group and 521 in the placebo group). Four of the 1063 patients were not included in the primary analysis because no postbaseline data were available at the time of the database freeze.
PRIMARY OUTCOMES
The primary outcome measure was the time to recovery, defined as the first day, during the 28 days after enrollment, on which a patient satisfied categories 1, 2, or 3 on the eight-category ordinal scale. Patients in the remdesivir group had a shorter time to recovery than patients in the placebo group (median, 11 days, as compared with 15 days; rate ratio for recovery, 1.32; 95% confidence interval [CI], 1.12 to 1.55; P<0.001; 1059 patients. Below is a summary of primary outcomes:
- Not hospitalized, no limitations of activities;
- Not hospitalized, limitation of activities, home oxygen requirement, or both;
- Hospitalized, not requiring supplemental oxygen and no longer requiring ongoing medical care (used if hospitalization was extended for infection-control reasons);
- Hospitalized, not requiring supplemental oxygen but requiring ongoing medical care (Covid-19–related or other medical conditions);
- Hospitalized, requiring any supplemental oxygen;
- Hospitalized, requiring noninvasive ventilation or use of high-flow oxygen devices;
- Hospitalized, receiving invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO); and
- Death.
The results are summarized below, highlighting the only group that showed a statistically significant improvement in outcomes as a result of taking the drug vs placebo.
SAFETY OUTCOMES
Serious adverse events occurred in 114 patients (21.1%) in the remdesivir group and 141 patients (27.0%) in the placebo group (Table S3); 4 events (2 in each group) were judged by site investigators to be related to remdesivir or placebo. There were 28 serious respiratory failure adverse events in the remdesivir group (5.2% of patients) and 42 in the placebo group (8.0% of patients). Acute respiratory failure, hypotension, viral pneumonia, and acute kidney injury were slightly more common among patients in the placebo group. No deaths were considered to be related to treatment assignment, as judged by the site investigators.
The NEJM said that “the lack of benefit seen in the other groups might have stemmed from a smaller number of patients in each group.” Still, as a result of the partial benefit for patients in the supplemental oxygen group, the study from the National Institute of Allergy and Infectious Diseases was evaluated early and led to the authorization of remdesivir before the full trial was completed.
Our findings highlight the need to identify Covid-19 cases and start antiviral treatment before the pulmonary disease progresses to require mechanical ventilation.
Overall, the study shows that whereas there was a modest benefit only to patients who were receiving oxygen, the results were statistically insignificant vs placebo for patients not receiving oxygen, while in a surprising twist patients on high-flow oxygen or mechanical ventilator/ECMO did modestly better in the placebo group than those taking remdesivir.
“Remdesivir was superior to placebo in shortening the time to recovery in adults hospitalized with Covid-19 and evidence of lower respiratory tract infection,” study concludes.




