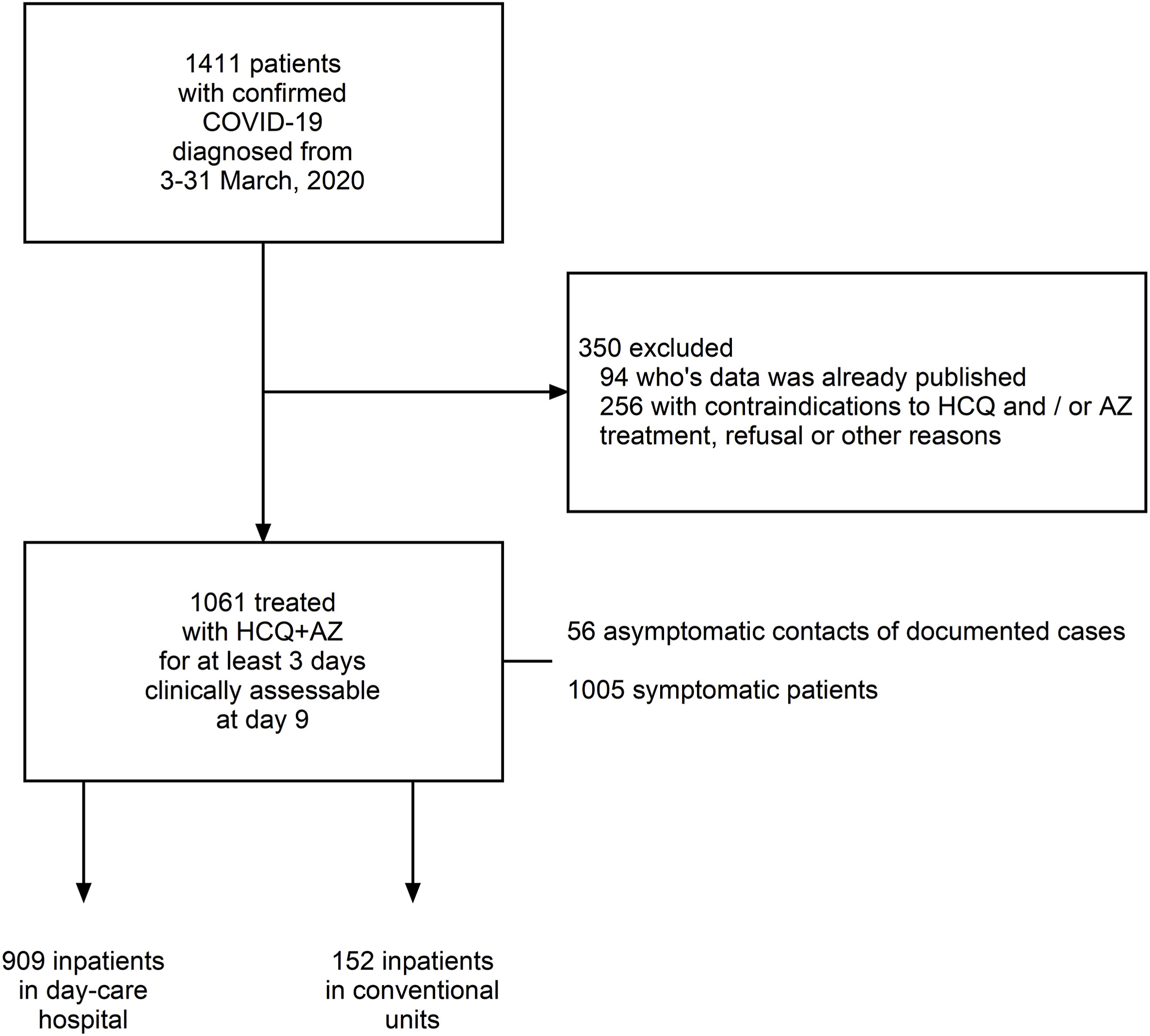
A new French study of 1061 patients found that early treatment of coronavirus patients with hydroxychloroquine and azithromycin is safe with low fatality rate (99.25% success)

We’ve written a lot of articles about hydroxychloroquine and other promising drugs currently being used to treat the deadly coronavirus. However, hydroxychloroquine has turned out to be the most controversial drug partly because the drug was touted by President Trump as a game-changer.
Despite several anecdotal evidences from doctors and COVID-19 patients that claimed the drug to be effective, other studies showed the malaria drug is not fully effective in protecting against coronavirus. FDA even cautioned against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting due to risk of heart rhythm problems. Another related-study found the results of the randomized clinical trial of 81 COVID-19 patients show that high-dosage of chloroquine with azithromycin was not sufficiently safe to warrant continuation of the study.
However, a new French study by Prof. Didier Raoult and his team of scientists, showed quite the opposite result. The study, which provides retrospective analysis of the treatment of 1061 patients with hydroxychloroquine and azithromycin, found that early treatment of COVID-19 patients with hydroxychloroquine and azithromycin is safe and has very low fatality rate in patients. The study is published in Travel Medicine and Infectious Disease journal.
According to the study, a total of 1061 COVID-19 patients were treated with 200 mg of hydroxychloroquine three times daily for ten days plus 500 mg azithromycin on day 1 followed by 250 mg daily for the next four days for at least three days. Of the 1061 patients included in this analysis, 8 patients died (0.75%) (74–95 years old) and a poor clinical outcome (PClinO) was observed for 46 patients (4.3%). All deaths resulted from respiratory failure and not from cardiac toxicity. Five patients are still hospitalized (98.7% of patients cured so far).
A preliminary French non-randomized clinical trial conducted in 36 COVID-19 patients showed a significant reduction in viral nasopharyngeal carriage at day 6 in patients treated with HCQ at 600 mg per day during 10 days (N = 20, 70% testing negative), compared to untreated controls (N = 16, 12.5% testing negative). In addition, of the twenty patients who were treated with HCQ, six received AZ for five days (for the purpose of preventing bacterial super-infection) and all (100%) were virologically cured at day 6, compared to 57.1% of the remaining 14 patients The synergistic effect is the rationale to use the combination HCQ and AZ.
Below is the abstract of the study. You can read the detail here.
Background
In France, the combination hydroxychloroquine (HCQ) and azithromycin (AZ) is used in the treatment of COVID-19.
Methods
We retrospectively report on 1061 SARS-CoV-2 positive tested patients treated with HCQ (200 mg three times daily for ten days) + AZ (500 mg on day 1 followed by 250 mg daily for the next four days) for at least three days. Outcomes were death, clinical worsening (transfer to ICU, and >10 day hospitalization) and viral shedding persistence (>10 days).
Results
A total of 1061 patients were included in this analysis (46.4% male, mean age 43.6 years – range 14–95 years). Good clinical outcome and virological cure were obtained in 973 patients within 10 days (91.7%). Prolonged viral carriage was observed in 47 patients (4.4%) and was associated to a higher viral load at diagnosis (p < .001) but viral culture was negative at day 10. All but one, were PCR-cleared at day 15. A poor clinical outcome (PClinO) was observed for 46 patients (4.3%) and 8 died (0.75%) (74–95 years old). All deaths resulted from respiratory failure and not from cardiac toxicity. Five patients are still hospitalized (98.7% of patients cured so far). PClinO was associated with older age (OR 1.11), severity at admission (OR 10.05) and low HCQ serum concentration. PClinO was independently associated with the use of selective beta-blocking agents and angiotensin II receptor blockers (p < .05). A total of 2.3% of patients reported mild adverse events (gastrointestinal or skin symptoms, headache, insomnia and transient blurred vision).
Conclusion
Administration of the HCQ+AZ combination before COVID-19 complications occur is safe and associated with very low fatality rate in patients.




