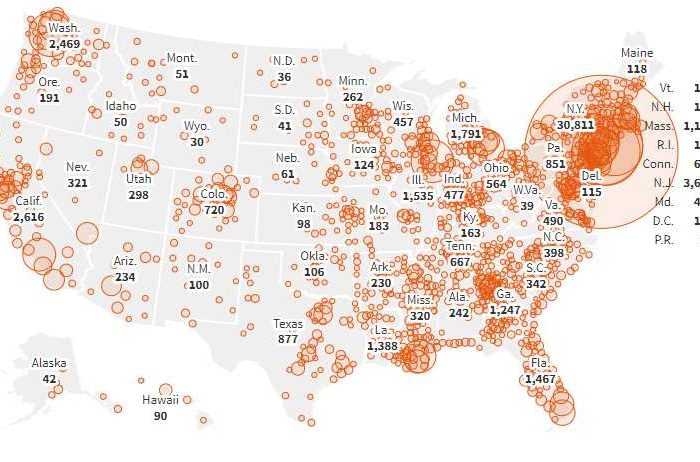
Israeli pharmaceutical company Teva to donate 6 million hydroxychloroquine sulfate tablets to hospitals nationwide for treatment of coronavirus (COVID-19)
Teva, an Israeli pharmaceutical company, announced it will more than 6 million doses of hydroxychloroquine sulfate tablets through wholesalers to hospitals across the U.S. to meet the urgent demand for the medicine as an investigational target to treat COVID-19. The company also pledged to donate 10 million more tablets within a month.
“We are committed to helping to supply as many tablets as possible as demand for this treatment accelerates at no cost,” said Brendan O’Grady, Teva Executive Vice President, North America Commercial. “Immediately upon learning of the potential benefit of hyroxychloroquine, Teva began to assess supply and to urgently acquire additional ingredients to make more product while arranging for all of what we had to be distributed immediately.”
Additional production of hydroxychloroquine sulfate tablets is also being assessed and subsequently ramped up with materials that are being sent to Teva from our ingredient supplier. Teva will ship 6 Million tablets through wholesalers to hospitals by March 31, and more than 10 Million within a month.
Hydroxychloroquine sulfate tablets manufactured by Teva are approved by U.S. Food and Drug Administration (FDA) for the treatment of malaria, lupus erythematosus and rheumatoid arthritis. Although the product is not currently approved for use in the treatment of COVID-19, it is currently under investigation for efficacy against the coronavirus and has been requested by US government officials to be made available for use immediately. The Company is also reviewing supply of both hydroxychloroquine and chloroquine globally to determine whether there are additional supply and access opportunities for patients.
Teva is also actively looking across its expansive range of products to determine if the company can help to provide any other products that may be relevant in addressing acute and substantial need during the COVID-19 crisis.