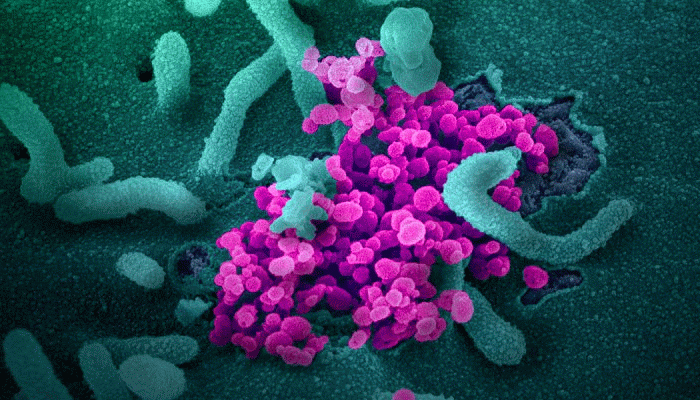
Researchers launched the first coronavirus COVID-19 vaccine trial in the U.S., Federal official says
As we reported on March 6, Kaiser Permanente Washington Health Research Institute announced it has received a green light from the government to begin its vaccine trials, the first of its kind. Its research team is enrolling 45 healthy people, ages 18 to 55, from the Seattle-area over the course of 14 months.
Today, the federal government announced today that the researchers has successfully administered the first shot in a trial for a potential vaccine for the novel coronavirus. The trial, which is taking place at the Kaiser Permanente Washington Health Research Institute in Seattle, aims to enroll 45 healthy adults over a six-week period, according to the National Institutes of Health (NIH).
“[The trial] does not include any form of the live virus, and the trial will not expose participants to the virus,” said Rebecca Hughes, senior media consultant with Kaiser Permanente. The trial is part one of three-phases that will study the safety of the vaccine and how well the immune system responds to it.
As part of the phase one trial, study participants will receive two doses of the vaccine approximately 28 days apart. The vaccine was developed by the NIH and its collaborators at Moderna Inc., based in Cambridge, Mass. It took only about two months for Moderna to develop the vaccine and ship it to the NIH. While the trial launched in record speed, public health officials have been stressing for weeks that a vaccine won’t be ready for 12 to 18 months in the best circumstances. The investigational vaccine has shown promise in animal models, and this is the first trial to examine it in humans.
“Finding a safe and effective vaccine to prevent infection with [the novel coronavirus] is an urgent public health priority,” National Institute of Allergy and Infectious Diseases Director Anthony Fauci said in a statement Monday. “This Phase 1 study, launched in record speed, is an important first step toward achieving that goal.”
The vaccine is the first to be tested in humans and is similar to the mRNA vaccines developed for the Zika virus.
Participants will receive $100 for each of the in-person study visits. People who complete every visit will get $1,100.