Health tech startup EBR Systems lands $30 million to prepare for commercialization of its WiSE Cardiac Resynchronization Therapy (CRT) System, the world’s only wireless cardiac pacing system
EBR Systems, a health tech startup and the developer of the world’s only wireless cardiac pacing system for heart failure, has closed $30M in new funding to complete enrollment of its pivotal SOLVE CRT clinical trial and to prepare for commercialization of the WiSE Cardiac Resynchronization Therapy (CRT) System. This financing was led by Australian private equity firms Brandon Capital Partners and M.H. Carnegie & Co., with participation from existing investors Split Rock Partners, Ascension Ventures, and Dr. Thomas Fogarty’s Emergent Medical Partners, among others. In conjunction with the funding, The company also announced John McCutcheon has joined EBR Systems as President and CEO.
Founded in 2003 by Rick Riley, the Sunnyvale, California-based EBR Systems develops devices for the treatment of cardiac arrhythmias. Heart failure is a serious condition in which the heart is unable to pump enough blood to meet the body’s demands. A progressive, debilitating disease, heart failure often occurs when electrical signals within the heart are disrupted, causing the heart’s ventricles to beat in an uncoordinated or unsynchronized pattern. That enlarges the left ventricle and makes the heart less efficient.
Cardiac resynchronization therapy is a proven treatment that improves symptoms and reduces hospitalizations and mortality1,2 by electrically stimulating the heart. Also referred to as biventricular pacing, CRT uses wire leads to synchronize the left and the right ventricles so that the two chambers beat together, thereby improving the heart’s efficiency. Approximately 200,000 patients are treated worldwide each year with traditional CRT. 3
SOLVE CRT is EBR’s multi-center, randomized, double blinded, prospective international study intended to assess safety and efficacy of the WiSE™ (Wireless Stimulation Endocardially) pacing technology in support of U.S. Food and Drug Administration (FDA) premarket approval (PMA) application. The study is enrolling 350 heart failure patients who have failed to respond to, or are otherwise unable to receive, conventional CRT. It is expected to complete enrollment in 2020.
“We are encouraged by the results and patient benefit shown in previous studies using the WiSE™ System and as such, we are excited to be continuing to support EBR Systems to bring this important technology to the market,” said Dr Chris Nave PhD, Managing Partner at Brandon Capital Partners. “EBR’s technology promises to revolutionize cardiac resynchronization therapy.”
“I’m delighted to join the fantastic team at EBR Systems to bring the unique WiSE System to the market and help improve patient outcomes,” said John McCutcheon, President and CEO of EBR Systems. “I look forward to guiding the organization through the crucial next steps of completing the study, gaining regulatory approvals, scaling manufacturing, and beyond.”
Approximately 30 percent of patients receiving conventional CRT do not respond to the therapy. A major cause of this shortcoming is believed to be the inconsistency of results achieved using wire leads to pace the left ventricle from within the coronary sinus vein on the epicardial (exterior) surface of the heart. Although it is generally accepted that stimulation of the left ventricle is preferable from inside the heart (endocardially), wire leads placed inside the left ventricle can cause clots, heart attacks or strokes. Additionally, wire leads can break, dislodge or otherwise fail, leading to complications in roughly 12 percent of cases.4 The financial impact of these issues is profound. More than $1 billion of $3.5 billion spent annually on CRT devices provides no patient benefit.5
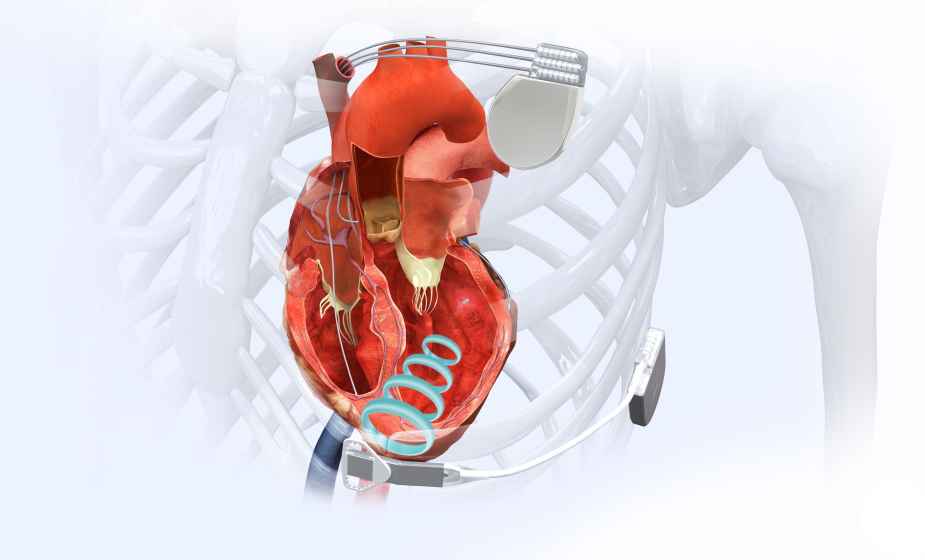
EBR Systems’ WiSE CRT System is the world’s only wireless, endocardial (inside the heart) pacing system in clinical use for stimulating the heart’s left ventricle. This has long been a goal of cardiac pacing companies since internal stimulation of the left ventricle is thought to be a potentially superior, more anatomically correct pacing location. WiSE Technology enables cardiac pacing of the left ventricle with a novel cardiac implant that is roughly the size of a large grain of rice. The need for a pacing wire on the outside of the heart’s left ventricle – and the attendant problems – are potentially eliminated.
The WiSE™ CRT System is CE Mark approved. In the US, it is an investigational device and not available for sale. The company’s patented proprietary Wireless Stimulation Endocardially (WiSE) technology was developed to eliminate the need for cardiac pacing leads, historically the major source of complications and reliability issues in cardiac rhythm disease management.